





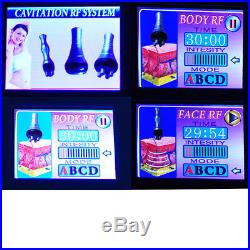







Release 40, 000 Hz ultrasonic, speedy vibrate fattiness cells, produce countless vacuum. Then takes the power of RF wave with 0.5 MHz, to do deep -seated skin Diathermy, supply. Lymph circulation, activate metabolism, remove and soften the cellulite, so get the body. Cells membrane, act on the cellulite, breaks them, then exhaust out of the body by. The fatness and produce explosion, during the course, no any damage to the blood vessels, nerves and tissues. Comfortable, painless, during the treatment, safety. Cells, and produce numerous vacuum cavitation, strong impact fat cells, so that fat cells. The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606. The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) NumberK132989. The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) NumberK180353. The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) NumberK141973. Massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) NumberK161892. The item “USA 3-1 Ultrasonic Cavitation RF Radio Frequency Vacuum Cellulite Slim Machine” is in sale since Friday, April 3, 2020. This item is in the category “Business & Industrial\Healthcare, Lab & Dental\Medical & Lab Equipment, Devices\Aesthetic & Cosmetic Machines”. The seller is “sinoshine016″ and is located in Los Angeles. This item can be shipped to United States.
- Brand: Unbranded
- MPN: Does Not Apply
- Country/Region of Manufacture: China
- UPC: 190891403438
- EAN: Does not apply
- 510(k) Number: K161892
